4. THE CHEMICAL NATURE OF THE FLAVOUR OF FRUIT BODIES OF MUSHROOMS
4. 1. Brief literature review
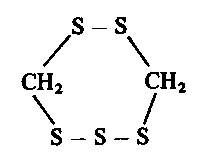
In 1966 Morita and Kobayashi [107] reported the isolation of lenthionine from fruit bodies of Lentinus edodes. It was the aroma-bearing substance of this mushroom and had the following unusual structure:
Furthermore guanosine-5'-monophosphate (5'-GMP) was shown to be an important flavour enhancing factor in Lentinus edodes. This nucleotide and, to a smaller extent, adenosine-5'-monophosphate (5'-AMP) stimulate the flavour of amino acids, especially glutamic acid, although they have little flavour when tasted alone [62,86].
The flavour of Lentinus edodes, fruit bodies of which are grown on a large scale in Japan, differs completely from the flavour of other edible mushrooms such as Agaricus bisporus, Boletus edulis and Coprinus comatus. An important aroma compound in the latter mushrooms is 1-octen3-ol. This compound was first isolated from Armillaria matsutake by Murahashi [111]. Thirty years later Freytag and Ney [46], isolated it from Agaricus bisporus. In both mushrooms the alcohol was optically active (leavorotatory).
In recent years several authors have investigated volatile products of mushrooms, using combined gas chromatography and mass spectrometry. Their results and those obtained in our investigations on Coprinus comatus are summarised in Table 20. In addition to the volatiles listed in Table 20, Thomas [142] identified in dried fruit bodies of Boletus edulis about 60 compounds including nine pyrazines, seven 2-formyl-pyrroles, six fatty acids (C5 to C10), five furan derivatives, four lactones and four aliphatic ketones. Many of these compounds may have been formed during the drying process. Dudareva [32] found in fresh Boletus edulis benzaldehyde, 3-heptanone (both listed in Table 20), isovaleric aldehyde, acetaldehyde, methyl cyclohexanone and four unidentified compounds.
Ethylene as well as low boiling volatiles (acetaldehyde, acetone, ethanol and ethyl acetate) and short-chain fatty acids (acetic, isobutyric, isovaleric and n-butyric acids) have also been found in Agaricus bisporus [97,134].
Concerning the non-volatile constituents of Agaricus bisporus several reports are available on the amino acid [2], nucleotide [84] and carbohydrate content [71], only the most recent works being cited here. Craske and Reuter [23] identified several amino acids in Boletus edulis.
Only a few reports discuss the organoleptic properties of the compounds identified. The only substances reported to have a mushroom odour are 1-octen-3-one and 1-octen-3-ol [24]. Craske and Reuter [23] stated that the characteristic flavour of Boletus edulis is associated with the basic amino acid fraction. The short-chain fatty acids [134], glutamic acid [59] and carbohydrates [93] have been suggested as contributing to the flavour of mushrooms, but without any experimental evidence.
Some patents (see note 1) about mushroom flavours have appeared. Nobukuni et al. [114] used 1,2,4,5-tetrathiohexane as a mushroom flavouring. Bentz and Mezzino [13] claimed that a mixture of 2,4,5-trimethyloxazole and diacetyl imparted an earthy, potato-like or mushroom-like flavour to foodstuffs. It is not clear from the patents concerned whether these substances have been identified in mushrooms. The sulphur containing compound 1,2,4,5-tetrathiohexane probably imitates the flavour of Lentinus edodes. Henriquez [69] reported that non-volatile derivatives of 1-octen-3-one and cis-4-heptenal could be prepared by reaction of the carbonylgroup with thiazolidine-4-carboxylic acid. The derivatives were added to dried soup preparations. On heating the rehydrated soup, 1-octen-3-one and cis-4-heptenal were liberated, giving the soup a mushroom odour.
Because, as is evident from the foregoing, many compounds have already been identified in Agaricus bisporus, our investigations were restricted to a quantitative analysis and an evaluation by a taste panel of the flavour significance of the known constituents of Agaricus bisporus.
Nothing has been published so far on the flavour of Coprinus comatus. Therefore, a chemical investigation of the constituents of this mushroom was undertaken, followed by an organoleptic evaluation. For comparison, some flavour compounds were analysed in a number of other edible mushrooms.
4.2. Organoleptic significance of constituents of Agaricus bisporus
An aqueous extract of Agaricus bisporus fruit bodies was prepared for enabling comparison of the results of chemical and organoleptic analyses of the same extract. Volatiles and fatty acids were determined in this extract by gas chromatography and nucleotides by ion exchange chromatography or enzymatic methods (for details, see Chapter 6.6.1). Using the results of our own analyses of L-glutamic acid, L-aspartic acid, glucose, reducing sugars and total sugars as a basis for comparison, the concentrations of other amino acids and carbohydrates were estimated from the data of Altamura et al. [2] and Holtz [71]
The results of the chemical analyses and the estimations mentioned are shown in Table 21. The compounds listed represent about 40% of the dry matter of the extract.
|
Table 21. Composition of a synthetic mixture approximating the natural extract of fruit bodies of Agaricus bisporus. The concentrations of amino acids and carbohydrates, not analysed in the present work, are based on the data of Altamura et al. [2] and Holtz [71] |
|||
|
Volatiles |
(μg/l) |
Amino acids (continued) |
(mg/l) |
|
(-)-1-octen-3-ol* |
4.3 |
L-2,4-diaminobutyric acid |
11.3 |
|
benzaldehyde* |
0.3 |
L-canavanine |
98.7 |
|
benzyl alcohol* |
6.5 |
creatinine |
894 |
|
butyric acid* |
2.0 |
|
|
|
isovaleric acid* |
0.5 |
5'-Nucleotides |
(mg/l) |
|
|
|
AMP* |
15.3 |
|
Amino acids |
(mg/l) |
ADP* |
44 |
|
L-aspartic acid* |
25.0 |
ATP* |
8.0 |
|
L-threonine |
32.5 |
GMP* |
31.2 |
|
L-serine |
39.3 |
UMP* |
26.0 |
|
L-asparagine |
19.4 |
UDPAG* |
141 |
|
L-glutamine |
21.5 |
|
|
|
L-glutamic acid* |
136 |
Carbohydrates |
(g/1) |
|
L-proline |
115 |
glucose* |
0.76 |
|
L-alanine |
140 |
saccharose |
0.17 |
|
L-2-amino-adipic acid |
24.0 |
mannitol |
3.83 |
|
glycine |
11.6 |
fructose |
0.16 |
|
L-cystine |
74.4 |
total sugars* |
1.10 |
|
L-valine |
36.3 |
reducing sugars* |
0.80 |
|
L-isoleucine |
40.1 |
|
|
|
L-leucine |
59.5 |
Miscellaneous |
|
|
L-phenylalanine |
25.4 |
urea* |
0.67 g/l |
|
3-amino-butyric acid |
111 |
ammonia* |
12.4 mM |
|
|
|
pH adjusted with KOH to |
6.1 |
|
*Actual analysis |
|||
Some organoleptic properties of the mushroom extract and synthetic mixtures that are relevant to flavour intensity are reported in Tables 22, 23, 24, 25 and 26. Distillation of the extract showed (Table 22), that the flavour of the mushroom extract was partly volatile; however, the flavour intensity of the non-volatile residue was at least two times stronger. A significant loss of flavour intensity was observed during this distillation.
a 95 % confidence.
The most important volatile aroma compound was (-)-1-octen-3-ol, with a flavour threshold concentration of 0.43 μl/l (Table 23). As the concentration of this substance in the mushroom extract was 4.3 μl/l, it accounts for 10.0 flavour units, which is comparable to the 11.1 flavour units found in the distillate of the extract (Table 22; for a definition of the threshold concentration and flavour units, see Chapter 6.6.2). Therefore, (-)-1-octen-3-ol can be considered as by far the most important volatile flavour compound in Agaricus bisporus. The flavour of the naturally occurring (-)-1-octen-3-ol is significantly stronger than the flavour of the (+)-isomer (Tables 24 and 25).
The flavour threshold concentration of 1-octen-3-one was at least ten times lower than the threshold concentration of the corresponding alcohol (Table 23) and of the same order of magnitude as the sensitivity of the gas chromatographic method, by which it could not be detected in the mushroom extract. Also Wasowicz [153] did not find 1-octen-3-one, while Picardi and Issenberg [121] found it only after boiling the mushrooms for at least 15 min. So if present, this compound does not contribute much to the flavour intensity, unless sub-threshold effects are important. Nor did the panel like its metallic flavour, which should probably be considered as an off-flavour, just as in dairy products [133].
a ln
water, 1.5 μl/l
b ln water, composition as in Table 21 (concentrations
x 1/3).
c In water, concentrations as in Table 21.
d See Chapter 6.6.3.
|
Table 25. The effect of modifications of the synthetic mixture (composition, see Table 21, concentrations x 1/6) on the flavour intensity. |
||||
|
Modification |
Flavour of original mixture stronger? |
|||
|
Yes |
No |
Modification stronger |
pa |
|
|
(-)-1-octen-3-ol replaced by (+)-1-octen-3-ol |
10 |
0 |
3 |
5.0 |
|
nucleotides omitted except GMP and AMP |
3 |
4 |
5 |
38.7 |
|
amino acids omitted except glutamic acid |
4 |
1 |
7 |
28.2 |
|
amino acids and nucleotides omitted except glutamic acid, GMP and AMP |
15 |
1 |
6 |
4.4 |
|
n-butyric and isovaleric acid omitted |
11 |
5 |
8 |
34.2 |
|
Benzaldehyde and benzyl alcohol omitted |
11 |
5 |
8 |
34.2 |
|
carbohydrates omitted |
12 |
10 |
2 |
3.3 |
a See Chapter 6.6.3.
The stimulating effect of nucleotides, having little flavour when tasted alone, on the flavour of amino acids as found by many workers (cited by Gutzeit-Walz and Solms [62] ) could also be observed in the systems studied in the present work (Tables 24 and 26). Among the flavour enhancing nucleotides GMP is known to have the greatest effect, followed by AMP [86]. The flavour of glutamic acid is stimulated most by nucleotides, but stimulation of the flavour of other amino acids, e.g. aspartic acid and methionine, has also been observed [62].
|
Table 26. Flavour intensity of synthetic mixtures (composition, see Table 21) expressed in flavour units. Numbers not followed by the same letter in the last column are significantly differenta. |
|||
|
|
Most probable number |
Confidence limits a |
|
|
Amino acids |
3.2 |
1.6 -6.4 |
A |
|
Nucleotides |
0.7 |
0.4 -1.4 |
B |
|
Amino acids and nucleotides |
8.9 |
3.9 -20.4 |
AC |
|
Carbohydrates |
0.8 |
0.4 -1.4 |
B |
|
(-)-1-octen-3-ol |
10.0 |
5.3 -19.0 |
DC |
|
Amino acids, nucleotides, carbohydrates and (-)-1-octen-3-ol |
46.5 |
30.0 -72.1 |
E |
|
Complete mixture (composition, see Table 21) |
70.2 |
39.0 -126.4 |
E |
|
Natural extract of Agaricus bisporus (taken from Table 22) |
62.5 |
48.1 -81.3 |
E |
a 95 % Confidence.
Table 25 shows that omission of all amino acids, except glutamic acid, did not decrease the flavour intensity of the mixture; nor did omission of all nucleotides, except GMP and AMP. However, omission of both amino acids and nucleotides, except glutamic acid, GMP and AMP, resulted in a decrease in flavour intensity, although the result was just at the significant level. Nucleotides are also known to enhance the flavour of octanal, an aroma compound of citrus fruits [128]. We could not demonstrate a similar effect on (-)-1-octen-3-ol, nor on the carbohydrates present in Agaricus bisporus (Table 24). The significant decrease of the flavour intensity during distillation (Table 22) is possibly due to decomposition of nucleotides. Omission of carbohydrates from the synthetic mixture resulted in a significant decrease in flavour intensity (Table 25).
Isovaleric and n-butyric acid could not be tasted in water in the concentrations occurring in the mushroom extract, and omission of these acids from the synthetic mixture did not result in a significant decrease of the flavour intensity (Tables 24 and 25). Similar observations were made with benzaldehyde and benzyl alcohol.
The low-boiling volatiles (acetaldehyde, acetone, ethanol and ethyl acetate) could not be detected in the aqueous extract. Their concentrations must be so low (< 2 μl/l) that they have no important influence on the flavour. The other non-acid volatiles reported to occur in Agaricus bisporus (furfural, 2-methyl-butanol, 1-octanol, 3-octanol, 3-octanone and 2-octen-l-ol) were all found, when detectable, in smaller amounts than benzaldehyde.
There was no significant difference between the flavour intensities of the synthetic mixture and the natural mushroom extract (Table 26), but the two flavours were not identical. Most of the panel members described the flavour of the synthetic mixture as "mushroom-like, synthetic". This might be due to the absence of hitherto unknown compounds, or to the absence of the known trace compounds that were omitted.
It can be concluded from the above observations, that the flavour of Agaricus bisporus is a complex phenomenon in which (-)-1-octen-3-ol plays an important role. Nucleotides, amino acids and carbohydrates also contribute significantly. The other compounds found in Agaricus bisporus do not have much quantitative influence on the flavour, but they may modify its quality.
4.3. Flavour compounds in Coprinus comatus
Volatile compounds in an aqueous extract of fruit bodies of Coprinus comatus were analysed by gas chromatography and mass spectrometry. Fig. 9 and Table 27 show the results. The compounds identified were previously compared with the volatile constituents of other mushrooms (Table 20). The presence of a group of C8-alcohols and ketones (1-octanol, 3-octanol, 3-octanone, 1-octen-3-ol) is a common property of Agaricus bisporus, Boletus edulis and Coprinus comatus, while 2-octen-1-ol is also present in Agaricus bisporus and Boletus edulis. The flavour threshold concentrations of these compounds are of the same order of magnitude as, or lower than, the concentrations in which they occur in the extract of Coprinus comatus (Table 28), so that they may influence the flavour. Of the other volatiles of Coprinus comatus only 2-methyl-2-penten-4-olide which, as far as we know, was not found earlier in natural material, has a sufficiently low threshold concentration to be important to the flavour. 4-Methyl-2-penten-4-olide, an isomer of the lactone of Coprinus comatus, has been found with other lactones in lavender oil [143].
The configuration of flavour compounds should not be neglected, because it may influence the flavour, as has already been shown in tests of 1-octen-3-ol. Flavour differences between the L- and D-isomers of amino acids have also been reported [131]. Aqueous solutions of L-leucine, L-phenylalanine, L-tyrosine and L-tryptophane (0.3%; pH 6) had a bitter taste, while the corresponding D-isomers were sweet.
|
Table 27. Identification of peaks shown in Fig. 9. |
||
|
Peak no |
Identification |
Techniques |
|
1 - 7 |
hydrocarbons a |
MS |
|
8 |
3-octanone |
MS GCb IR |
|
9 |
C3-alkylbenzene |
MS |
|
10 |
3-octanol |
MS GC |
|
11 |
1-octen-3-ol |
MS GC IR |
|
12 |
hydrocarbon |
MS |
|
13 |
C15-alkane a |
MS |
|
14 |
1-octanol |
MS GC |
|
15 |
2-methyl-2-penten-4-olide |
MS GC IR NMR |
|
16 |
hydrocarbon a |
MS |
|
17 |
Cl7-alkane |
MS |
|
18 - 19 |
C18-hydrocarbons |
MS |
|
20 |
2,6-ditert.butyl-p-cresol a |
MS IR |
|
21 |
1-dodecanol |
MS GC IR |
|
22 |
dodecylacrylate a |
MS GC IR NRM |
|
23 |
caprylic acid |
MS GC |
a Peaks also found in blank extract.
b GC = comparing of GC-retention data of reference compounds.
a Approximate
flavour threshold concentration in μg/l.
b Tentative identification
based on retention data only.
In Coprinus comatus 1-octen-3-ol, 3-octanol and 2-methyl-2penten-4-olide may have optical activity, but the amounts of 1-octen-3-ol and 3-octanol were too small to enable it to be determined. When isolated in sufficient quantities from plants and fungi, these compounds were always identified as (-)-1-octen-3-ol [46] and (+)-3-octanol [67, 87,112]. The compounds are configurationally related, because catalytic hydrogenation of (-)-1-octen-3-ol leads to the formation of (+)-3-octanol [92]. The amount of 2-methyl-2-penten-4-olide in Coprinus comatus was sufficient for ORD-analysis. The absolute configuration of this lactone is shown in Fig. 9A.
Because only 1-octen-3-ol was available in an optically active form, 3-octanol and 2-methyl-2-penten-4-olide were tasted as the racemic mixture (see note 2). Thus the flavour threshold concentrations of the natural forms of 3-octanol and 2-methyl-2-penten-4-olide may differ somewhat from the data presented in Table 28.
Several non-volatile compounds (sugars, amino acids and nucleotides) were determined in the extract of Coprinus comatus by enzymatic methods or ion exchange chromatography; the results are included in Table 28. The amino acids found represent 20.4 mM amino nitrogen (62% of total amino nitrogen) and 2.67 g/1 of dry matter (19% of the dry matter of the extract). The total concentration of sugars, as measured by two methods, was high (73% of the dry matter of the extract). Hence fruit bodies of Coprinus comatus are rich in free amino acids and sugars.
The concentrations of glutamic acid and 5'-GMP in the extract were 3 to 4 times as high as in the extract of Agaricus bisporus studied earlier (Table 21). Since these substances are important to the flavour of Agaricus bisporus, it may be concluded that they also should contribute significantly to that of Coprinus comatus. The other amino acids, although not very important in Agaricus bisporus, may be present in Coprinus comatus in sufficient quantities to influence the flavour. Only a small part of the sugars was determined by enzymatic methods, and no alcohols like mannitol, which is present in high concentrations in Agaricus bisporus. Hence, no conclusions can yet be drawn about the influence of carbohydrates on the flavour of Coprinus comatus.
|
Table 29. Comparison of the flavour intensities of the natural extract of fruit bodies of Coprinus comatus and a synthetic mixture (composition, see Table 28). |
||
|
|
Flavour units |
Confidence limits (95%) |
|
natural extract |
31 |
17 - 58 |
|
synthetic mixture |
100 |
58 - 173 |
The flavour intensity of the mixture of 36 compounds identified in the extract of Coprinus comatus was compared with that of the natural extract (Table 29) and found to be significantly stronger. Furthermore, the two flavours were not qualitatively identical. Probably in the natural extract yet unknown flavour compounds, for instance sugars, partly mask the flavour of the compounds so far demonstrated analytically.
Although the full chemical composition of the flavour of Coprinus comatus has not been established it may be concluded that the amino acids, nucleotides and volatiles so far identified form an important part of it.
4.4. Flavour compounds in some other mushrooms
In Agaricus bisporus and Coprinus comatus 1-octen-3-ol is the only compound known to have a typical mushroom odour. The concentration in fresh Coprinus comatus is lower than in fresh Agaricus bisporus, while the concentrations of some other volatiles with intense odours are higher in the former species. This may explain the different flavours of the two mushrooms. In the non-volatile fractions 5'-GMP and glutamic acid are the most important flavour compounds.
For comparison the occurrence of 1-octen-3-ol, 5'-GMP and glutamic acid in other mushrooms was investigated (Table 30); canned and dried mushrooms were also examined. In order to find out whether the results obtained with such samples provided any information about the corresponding fresh mushrooms, the effect of sterilisation and drying on the concentrations of 1-octen-3-ol, 5'-GMP and glutamic acid in Agaricus bisporus was investigated. Table 30 shows that Agaricus bisporus lost 1-octen-3-ol and 5'-GMP during drying and to a smaller extent during sterilisation, which, in turn, must result in a loss of flavour. Hence the observation of low concentrations of 1-octen-3-ol and 5'-GMP in several canned and dried mushrooms, does not imply that the corresponding fresh mushrooms are equally poor in these flavour compounds. From the results of Thomas [142] and Wasowicz and Kaminski [154] it may also be concluded that there is much difference between the volatiles of dried and fresh Boletus edulis fruit bodies (Table 20).
Recent investigations [10] have shown that more than 80% of mushroom volatiles can be retained in a mushroom extract during freeze-drying if the initial concentration of solids is more than 5%.
The concentration of glutamic acid was increased moderately by sterilisation, possibly due to a breakdown of protein, and it was lowered somewhat on drying during several hours, perhaps because of Maillard reactions.
All extracts of fresh and canned mushrooms contained 1-octen-3-ol, but the concentrations varied considerably. Only in Cantharellus cibarius was the concentration of 1-octen-3-ol below the flavour threshold (0.43 pi/1) and in Tricholoma nudum the concentration was just at the threshold level. The other fresh and canned mushrooms contained so much 1-octen-3-ol that it may have an important influence on the flavour. Agaricus bitorquis contained 5.5 times as much 1 -octen-3-ol as Agaricus bisporus to which it is closely related. In this concentration 1-octen-3-ol greatly influences the flavour of Agaricus bitorquis.
Abbott and San Antonio [1] found that most tasters who had proved themselves able to distinguish Agaricus bitorquis from Agaricus bisporus preferred Agaricus bisporus. They further reported that several tasters found Agaricus bitorquis somewhat too strongly flavoured as a sautéed mushroom, but supposed that it would be suitable as a flavouring ingredient for other foods. These observations suggest that high levels of 1-octen-3-ol are less pleasant.
Pholiota squarrosa and Pleurotus ostreatus also contained fairly high concentrations of 1-octen-3-ol, and a very high quantity (440 x the threshold concentration) was found in the giant puffball, Calvatia gigantea. A gas chromatogram of a concentrated extract of this species (Fig. 10) showed, that 1-octen-3-ol is the dominant volatile, representing 89% of the volatiles found in the chromatogram.
The fruit bodies of the fresh mushrooms studied also contained 5'-GMP in varying concentrations. The highest amounts were found in Coprinus comatus and Pleurotus ostreatus. Rather low concentrations were observed in Lactarius sanguifluus, Pholiota squarrosa and Tricholoma nudum. The dried mushrooms, except Lentinus edodes and Agaricus bisporus, did not contain much 5'-GMP and none could be detected in the canned mushrooms. Canned and dried Boletus edulis and canned Cantharellus cibarius contained the smallest amounts of glutamic acid. In all other samples glutamic acid was present in sufficient quantities to have an important influence on the flavour, especially if much 5'-GMP is simultaneously present.
An extract of potatoes was analysed for comparison, because 1-octen-3-ol and 5'-GMP also occur in them [19,115]. The potatoes were similar to fresh mushrooms only as far as glutamic acid is considered.
The concentrations of 1-octen-3-ol and 5'-GMP are very low. It should be borne in mind that in the procedure applied only free nucleotides were determined. Buri [19] also found low concentrations of free mononucleotides in raw potatoes. The enzyme phosphodiesterase, which is able to hydrolyse nucleic acids, thus liberating 5'-nucleotides, is extremely heat stable. It has been found in several plants; only about 30% of the original activity was lost when the enzyme, isolated from Avena leave tissue, was incubated in 0.1 M tris-acetate buffer (pH 6.8) at 800 °C for 5 min [147]. Phosphodiesterase from carrot had almost the same activity at 37 °C and 60 °C [65]. Buri [19] showed that 5'-nueleotides were liberated enzymatically during the heating of potatoes, resulting in an amount of mononucleotides that was significant in the flavour.
In tomatoes 1-octen-3-ol could not be detected, while the concentration of 5'-GMP was low.
4.5. Discussion
Although the flavours of Agaricus bisporus, Agaricus bitorquis, Calvatia gigantea, Coprinus comatus, Pleurotus ostreatus, Pholiota squarrosa, Lactarius sanguifluus and Tricholoma nudum are not identical, 1-octen 3-ol, 5'-GMP and glutamic acid are present in all these mushrooms insufficient amounts to be important to the flavour. These compound probably form a basal flavour, which is modified in some species by the presence of other compounds.
The weak flavour of many canned and dried mushrooms is explained by their much lower concentrations of 1-octen-3-ol and 5 -GMP, compared with those of fresh mushrooms. An exception is Lentinus edodes this mushroom, when dried, still contains an important amount of 5' GMP, while the specific flavour compound lenthionine is also present in dried samples of the same mushroom [107].
Notes to chapter 4
Note 1. Just before this dissertation was printed we found some recent patents of Munekata and Imaki [165,166]. They described new methods for synthesising 2-octen-l-ol and 1-octen-3-ol. Both compounds were found to have a mushroom flavour.
Note 2. Later we also prepared synthetic (+)-3-octanol and (-)-3-octanol. With a small taste panel, we did not observe any difference between the flavours of these compounds. In this respect a remark of Pickard and Kenyon [122] is interesting. They observed, that the odours of the optically active alcohols were far weaker than those of the corresponding racemic mixtures. They further observed a slight difference between the odours of the (+)- and (-)-forms of some secondary alcohols, such as 1-phenyl-1-propanol.
References to chapter 4